Restoration Practices Knowledge Base
5.1 What to restore?

Before restoring forests and soils, it’s vital to understand their roles in ecosystem health and the impacts of forest degradation on soil. Forests and soils are tightly linked and should be managed as one system. Restoration is context-dependent, shaped by local conditions and land-use history. Here we propose a set of indicators for soil physical, chemical and biological properties, some of which are considered ‘scalable’, i.e. designed to be applicable across diverse ecosystems, cost-effective, and easy to implement.
Forests and forest soils are critical components of terrestrial ecosystems, delivering a wide range of ecosystem services, including carbon sequestration, climate regulation, nutrient cycling, and water filtration. Therefore, their conservation should be prioritized wherever feasible to maintain ecological function and long-term sustainability. In cases where conservation is no longer viable due to anthropogenic disturbance or natural degradation, ecological restoration becomes necessary. Restoration efforts should adopt a holistic approach that promotes the recovery of soil health, including soil physical, chemical, and biological properties, while recognizing the intrinsic interconnection between forest and soil restoration. To effectively guide restoration, it is essential to assess both the condition and the capability of soils through spatial mapping and site-specific diagnostics. This enables the selection of appropriate restoration strategies that align with soil constraints and potential. Furthermore, soil capability mapping can inform realistic expectations for ecosystem service recovery. Restoration itself can consist of multiple techniques and interventions, which are being summarized in this report along with their impact on different soil parameters. This practical tool allows selecting context-appropriate interventions that effectively support soil health recovery and ecosystem resilience. Monitoring is a critical component to guide and evaluate restoration success by using scalable indicators that are cost-effective, time-efficient and applicable across spatial scales. These indicators enable cross-site comparisons and long-term assessments. Mapping soil capability and condition prior to restoration allows for the identification of site-specific limitations and potentials. Monitoring should begin with a pre-restoration baseline to establish reference conditions and guide indicator selection. Additionally, identifying degradation problems is an important step in the pre-monitoring phase. Post-restoration monitoring must be structured, consistent, and longitudinal, capturing short- and long-term soil responses. The use of scalable indicators facilitates tracking of soil recovery across heterogeneous landscapes, improving comparability and policy relevance.
Despite clear definitions of soil health, they usually do not come with specific guidelines for how to measure or monitor it in practice (Fierer et al., 2021). Particularly, which variables are most relevant to assess the soil’s condition. It is clear that soil health cannot be defined by a single ‘optimal’ state, as it varies across ecosystems and land uses (Bünemann et al., 2018; Lehmann et al., 2020). Still, certain indicators are consistently useful for assessing soil health. For instance, healthy soils typically are well-structured (physical health/condition), contain sufficient SOC to bind water and nutrients (chemical health/condition) and are full of life (biological health/condition) (Frene et al., 2024; Raghavendra et al., 2020; Stewart et al., 2018; Wang & Zhang, 2024). Effective soil restoration addresses these three key categories of soil properties, i.e. physical, chemical, and biological (Raghavendra et al., 2020; Stewart et al., 2018). In this chapter, we propose a set of indicators for each category, some of which are considered ‘scalable’, i.e. designed to be applicable across diverse ecosystems, cost-effective, and easy to implement.
1. Physical properties
Within the physical properties we propose to evaluate bulk density and aggregate stability as they influence the interconnectivities between plants and soils and can be influenced by human activities. Soil texture is also an important physical measure, yet as it is not variable under management it proxies more the soil’s capacity than its condition.
Bulk density, expressed in mg/cm³, is a key indicator of soil compaction and overall soil health, influencing water infiltration, root development, and carbon stock estimates (Lee et al., 2009; Panagos et al., 2024; Vogt et al., 2015). It measures the dry mass of soil per unit volume and varies with management practices (Al-Shammary et al., 2018). Sampling methods include direct (core, clod, excavation) and indirect (radiation, regression) approaches; while indirect methods are more accurate, they are also costlier and require specialized skills (Vogt et al., 2015). Consistent fixed-depth sampling - ideally five samples per stand - is recommended to account for variability with depth (Cools & De Vos, 2010). In the SUPERB project, bulk density was measured using the core method at 0-5 cm (100 cm³ Kopecky ring), and an 18 mm auger for 5-15, 15-40, and 40-80 cm layers (FunDivEUROPE, 2011). Samples were oven-dried at 105 °C, weighed, and bulk density was calculated as dry mass divided by sample volume.
Additionally, aggregate stability is an important indicator for soil health because it is related to erodibility and soil-water dynamics (Rieke et al., 2022). Rieke et al., 2022 evaluated four methods for aggregate stability and concluded that all four methods were viable options. Considering cost-effectiveness, method accessibility, and time efficiency, the slaking test, adapted from the SLAKES smartphone image recognition software, offers a practical and scientifically robust approach for assessing aggregate stability in the field. This method is also preferred by the Soil Health Institute.
2. Chemical properties
The chemical properties refer to the soil’s composition and reactions. These properties determine how well soil can supply essential elements to plants and other soil organisms and buffer against pollutants. Here we propose to measure total soil carbon, total soil nitrogen and soil pH. Other properties that could be interesting to evaluate the soil’s chemical condition are cation exchange capacity (CEC), as it reflects the soil’s ability to retain and supply nutrients, electrical conductivity (EC), as it indicates soil salinity, and the soils status for specific nutrients that plants need to grow (e.g. NO3-, NH4+, P, K, base cation contents).
Measuring soil carbon helps to track how much carbon forests are sequestering and how environmental changes affect this role. Additionally, soil carbon is closely linked to soil fertility, structure, water retention and biological activity. Withing SUPERB, sampling for carbon was through the same method as for bulk density (FunDivEUROPE, 2011). Soil carbon occurs in both organic molecules and inorganic carbonates. Different analysing techniques are used depending on the type of carbon being measured. Methods for determining total carbon are through dry combustion or wet oxidation (Vogt et al., 2015). Within SUPERB, total carbon was analyzed. Soil samples were dried and crushed to obtain finely ground soil and analysed with the Flash 2000 Organic Elemental. This analysis works via dry combustion (T = 950 °C) followed by gas chromatography (Robertson, 1999). When carbonates were present in the soil, first hydrochloric acid was added to the soil sample to only obtain the soil organic carbon.
Soil pH is perhaps the most important factor in soil fertility and a critical indicator of soil quality, significantly influencing forest health and ecosystem functioning by affecting various biological and chemical processes. It also plays a crucial role in the activity and diversity of soil bacteria and fungi and is essential for the specific pH preferences of different tree species. (O’Neill et al., 2005; Singh et al., 2011; Thomas, 1996; Vogt et al., 2015). The soil pH was sampled using the same method as for soil carbon and pH (FunDivEUROPE, 2011). Regarding lab analysis, there is no standard procedure for measuring pH and can vary from laboratory to laboratory. pH can be measured using rapid field methods or more precise laboratory techniques: pH-H2O and pH-KCL. pH-H₂O is determined by adding soil with water and measures only the free hydrogen ions (H⁺) in the soil solution. It does not account for hydrogen ions that are attached to clay and organic matter. The pH-KCl is determined by mixing soil with a potassium chloride solution. The potassium ions (K⁺) replace the hydrogen ions bound to clay and humus, so pH-KCl measures both the free and the exchangeable hydrogen ions in the soil (Van Ranst et al., 1999). Within SUPERB, pH-H2O was measured on a 1g:10mL soil:liquid extract for the forest floor samples and a 1g:5mL soil:liquid extract for the mineral soil samples (Van Ranst et al., 1999). These ratios also apply when using the pH-KCl method with KCl 1M.
3. Biological properties
Soil microbes break down the organic matter through catabolic processes. Additionally, they play a pivotal role in nutrient cycling, soil structure formation and overall soil fertility. Microorganisms are highly responsive to environmental changes, making them valuable indicators for assessing forest restoration. Various methods are available for determining soil microbial diversity, including culturing, microscopy, DNA-methods and image analysis (Vogt et al., 2015). Here, we focus on the potential catabolic activity/diversity and metabolic activity.
Assessing catabolic microbial activity and diversity is essential for understanding soil health. By evaluating potential catabolic activity, we can quantify the efficiency of microbial communities in mineralizing organic substrates, thereby releasing essential nutrients and contributing to the soil’s nutrient pool (Vogt et al., 2015). Within SUPERB, we selected the Biolog EcoPlate™ method to measure this parameter (Gaublomme et al., 2006). Microorganisms inoculated onto different carbon sources leave a response pattern over a time (Biolog, 2023). To ensure accurate assessment, samples were collected in a sterile way by using disposable gloves, with all equipment sterilized using ethanol 90% and a gas burner. Samples were stored under cool conditions (7°C) and analyzed withing 14 days (FunDivEUROPE, 2013). In the lab, the fresh samples were being diluted and pipetted onto the Biolog EcoPlates™, containing 31 different carbon sources and 1 control in triplicates. The inoculated plates were placed in an incubator at 25°C for a period of 48 hours. The absorbance was measured after filling the ecoplates (day 0) using a VERSAmax microplate reader (OD590 nm) and on day 3 and 5. With the obtained data the average well color development and the Shannon diversity index can be expressed.
The metabolic activity is a sensitive indicator of many belowground processes and ecological interactions. Soil respiration is an indirect index for soil biological activity and can be used to assess microbial biomass (Vogt et al., 2015). Soil microorganisms respire carbon dioxide as a byproduct of metabolism while degrading organic matter and cycling nutrients (Rieke, Cappellazzi, et al., 2022). Within SUPERB, the microbial biomass was measured by incubating rewetted dried and sieved soil (10-15 gram) in a jar during 24 hours at 24°C (Comeau et al., 2023; Moebius-Clune, 2016; Soil health institute, 2022; Vogt et al., 2015). After 24 hours, CO2 levels were measured using the LICOR 7810. The measurement was done by collecting the gas with a syringe and injecting the sample into the closed loop of the LICOR system (Comeau et al., 2023). The CO2 levels were calculated by using the volume of the closed loop and the CO2 concentration before and after the injection. A more cost-effective and simpler alternative for measuring carbon levels (compared to the LICOR) is the Checkpoint Dansensor.
Additionally, the biomass of the fine roots can also be determined. Fine roots are a key indicator of belowground productivity and nutrient uptake, as fine roots are primarily responsible for water and nutrient absorption. Measuring fine root biomass helps assess ecosystem functioning, soil-plant interactions, and the impact of environmental changes or restoration on root dynamics (Likulunga et al., 2022; Magalhães & Mamugy, 2020; Vogt et al., 2015). Within SUPERB, fine root sampling was conducted using the same methodology as for soil carbon (FunDivEUROPE, 2011). Because laboratory analysis of fine roots can be labor-intensive and time-consuming, we selected a less intensive sampling method to balance feasibility with data quality. For each sample, fine root picking was carried out for 5 minutes on the dried but unsieved soil, and fine root biomass was calculated by dividing the mass of the collected roots by the total volume of the dried sample (Likulunga et al., 2022; Magalhães & Mamugy, 2020).
Related resources
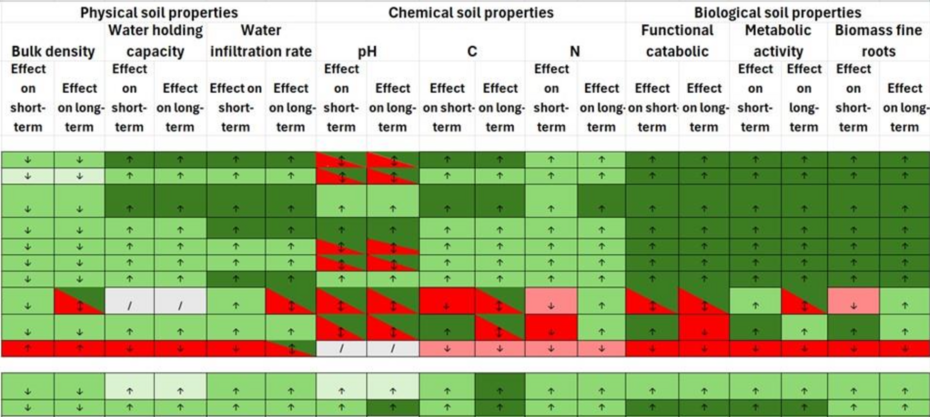
Forest and Soil Restoration Table
An Excel table that outlines various forest restoration measures and their effects on key soil parameters. This table offers a practical overview for assessing the benefits and limitations of different approaches to forest and soil restoration. Colours indicate a positive or negative effect, while the arrows indicate if there is an increase or decrease of the soil properties. When interpreting this table, it is important to consider that factors like previous land use and implementation quality can strongly influence soil recovery outcomes.
Soil Restoration and Monitoring Guidelines
Forests are essential ecosystems, covering approximately 35% of Europe’s land area. They harbour a significant share of Europe’s terrestrial biodiversity and provide a wide range of ecosystem services critical to European citizens. However, forests across Europe are increasingly threatened by various forms of degradation, driven by multiple, often anthropogenic factors.